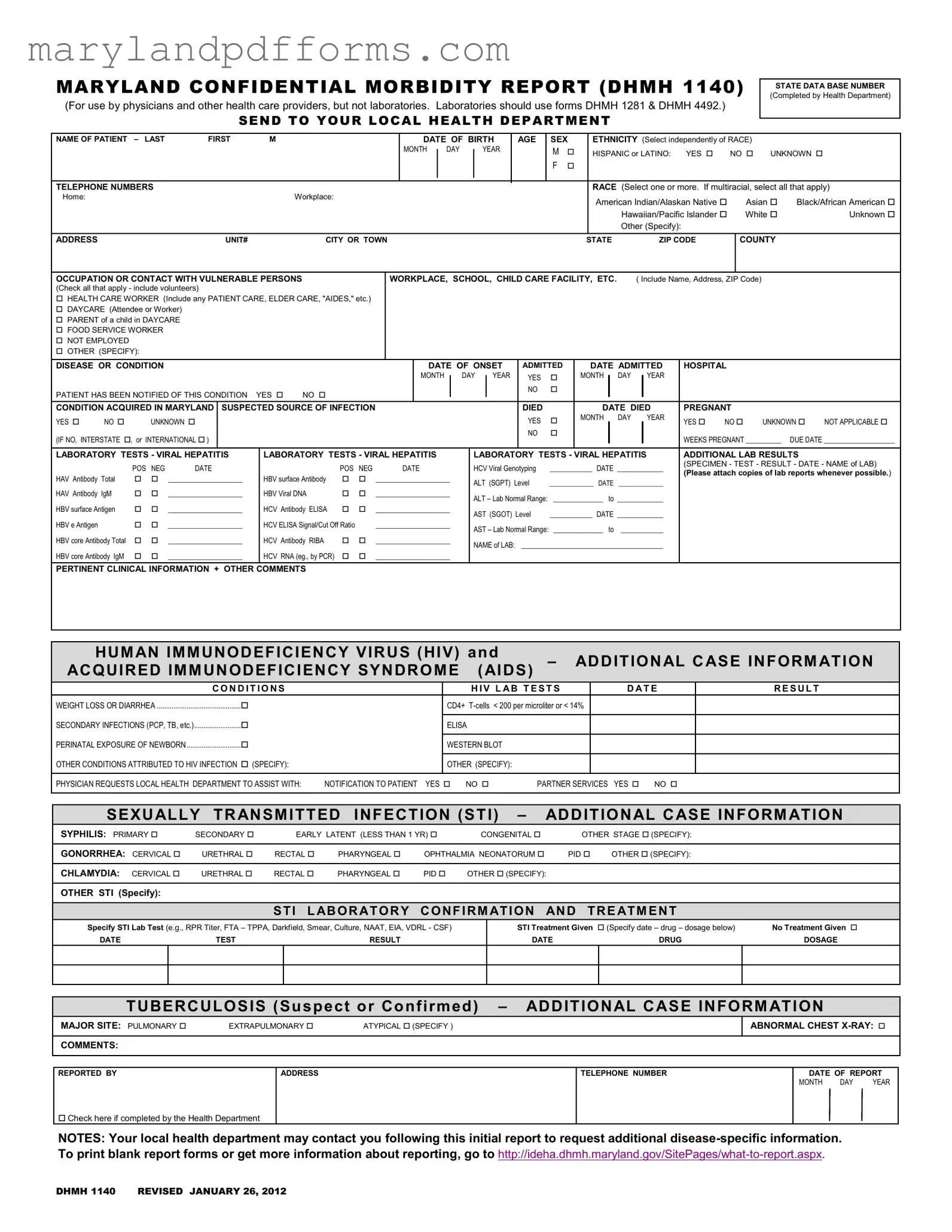
Blank Maryland Confidential Morbidity Report Template
Similar forms
- CDC Case Report Form: Similar to the Maryland Confidential Morbidity Report, the CDC Case Report Form collects detailed information on specific diseases. Both forms aim to track morbidity and mortality data to inform public health responses.
- Patient Health History Form: This form gathers comprehensive health information from patients, including demographics and medical history. Like the Maryland form, it focuses on understanding health conditions for better treatment and reporting.
- State Health Department Surveillance Form: This document is used by various states to monitor disease outbreaks. It shares similarities with the Maryland form in its purpose of disease tracking and public health intervention.
- Infectious Disease Reporting Form: Health care providers use this form to report cases of infectious diseases. Both forms require information about the patient and the disease, contributing to the overall understanding of public health trends.
- California Living Will Form: To document your healthcare preferences, utilize the structured California living will form instructions for ensuring your desires are honored.
- Birth and Death Certificates: These official documents record vital statistics. While they focus on births and deaths, they share the Maryland form's goal of collecting critical health data to inform health policies and programs.
Maryland Confidential Morbidity Report - Usage Steps
Completing the Maryland Confidential Morbidity Report form is a straightforward process. This form is essential for reporting specific health conditions and ensuring appropriate follow-up. Follow these steps carefully to ensure all necessary information is accurately provided.
- Begin by filling in the State Data Base Number. This will be completed by the local health department.
- Enter the patient's name in the format of last name, first name, and middle initial.
- Provide the date of birth, including month, day, and year.
- Indicate the age and sex of the patient.
- Select the ethnicity from the options provided. Choose independently of race.
- Fill in the telephone numbers for the patient, including home and workplace if applicable.
- Choose the race of the patient from the list. If the patient is multiracial, select all that apply.
- Provide the address of the patient, including unit number, city or town, state, and ZIP code.
- Indicate the county where the patient resides.
- Specify the occupation or any contact with vulnerable persons, checking all applicable boxes.
- Report the disease or condition being reported.
- Fill in the date of onset for the condition.
- Indicate if the patient was admitted to the hospital, including the date.
- Confirm whether the patient has been notified of this condition.
- State if the condition was acquired in Maryland and provide the suspected source of infection.
- Provide details regarding the patient's pregnancy status, including weeks pregnant and due date if applicable.
- Fill out the laboratory tests section, providing results and dates for relevant tests.
- Include any pertinent clinical information and additional comments.
- Indicate any requests for assistance from the local health department.
- Complete the sexually transmitted infection (STI) section, specifying the type and stage of STI.
- Provide details for any treatment given or specify if no treatment was provided.
- Complete the tuberculosis section if applicable, providing major site and comments.
- Finally, fill in the reported by section, including your address, telephone number, and the date of report.
Once the form is completed, it should be submitted to your local health department. Be prepared for potential follow-up requests for additional information. Accurate reporting is crucial for public health efforts.
Learn More on Maryland Confidential Morbidity Report
What is the Maryland Confidential Morbidity Report form?
The Maryland Confidential Morbidity Report (DHMH 1140) is a form designed for use by healthcare providers to report specific diseases and conditions. It helps local health departments track morbidity data and manage public health effectively.
Who should use this form?
This form is intended for physicians and other healthcare providers. Laboratories should use different forms, specifically DHMH 1281 and DHMH 4492.
What information is required on the form?
The form requires detailed patient information, including:
- Patient's name, date of birth, age, and sex
- Ethnicity and race
- Contact information
- Occupation and potential contact with vulnerable populations
- Details about the disease or condition, including onset date and hospitalization information
- Laboratory test results
How do I submit the form?
After completing the form, send it to your local health department. Ensure all required fields are filled out to avoid delays in processing.
Is patient consent required before reporting?
Yes, it is essential to notify the patient about the condition being reported. The form includes a section to indicate whether the patient has been informed.
What types of diseases or conditions should be reported?
The report covers various conditions, including:
- Viral hepatitis
- HIV/AIDS
- Sexually transmitted infections (STIs)
- Tuberculosis
Specific instructions for each condition are included on the form.
What happens after I submit the report?
Your local health department may contact you for additional information related to the reported case. This follow-up helps ensure accurate data collection and public health response.
Can I attach additional documents to the report?
Yes, you should attach copies of any relevant laboratory reports whenever possible. This additional information can be crucial for effective case management.
What should I do if I have questions about the form?
If you have questions, you can visit the Maryland Department of Health's website or contact your local health department for guidance. They can provide specific assistance related to reporting requirements.
Where can I find more information about reporting?
For more information about reporting and to access blank report forms, visit this link .
Additional PDF Forms
Fha Net Tangible Benefit - This worksheet is an essential part of the refinancing process in Maryland.
Md Pension Exclusion - Proper handling of sensitive information, such as social security numbers, is emphasized.
In order to ensure clarity and security in agreements associated with potential liabilities, it is beneficial to utilize resources like Templates Online, which offer templates for the Arizona Hold Harmless Agreement. This may help parties involved to navigate their responsibilities and avoid legal repercussions effectively.
Maryland Medicaid Application - Physicians must provide a detailed plan of care on the form.
Documents used along the form
The Maryland Confidential Morbidity Report form is an important document used by healthcare providers to report specific health conditions. Along with this form, several other documents may be necessary to ensure comprehensive reporting and management of health data. Below is a list of forms and documents often used in conjunction with the Maryland Confidential Morbidity Report.
- DHMH 1281 - This form is specifically for laboratory reporting of certain diseases. Laboratories must use this form to report cases that require laboratory confirmation.
- DHMH 4492 - Similar to DHMH 1281, this form is designated for laboratory use, focusing on reporting specific infectious diseases and conditions.
- Patient Consent Form - A document that obtains permission from patients to share their health information with local health departments or other entities for public health purposes.
- Clinical Laboratory Report - This report provides detailed results of laboratory tests performed on a patient, which may be required for further analysis of morbidity cases.
- Case Investigation Form - Used by health departments to gather detailed information about a reported case, including contact tracing and follow-up actions.
- Notification of Disease Form - A form that healthcare providers may use to notify health authorities about the occurrence of specific diseases or conditions in a patient.
- Follow-Up Report Form - This document is used to provide updates on a patient's condition after the initial morbidity report has been submitted.
- Immunization Record - A record of vaccinations received by the patient, which may be relevant in cases of infectious diseases.
- Referral Form - Used to refer patients to specialists or additional services, especially in cases of complex health conditions.
- Mobile Home Bill of Sale - Essential for the sale or transfer of ownership of a mobile home in New York, this form serves as proof of transaction and can be found at PDF Templates Online.
- Public Health Surveillance Report - A broader report that summarizes trends in morbidity and mortality data, often used for epidemiological studies.
These documents collectively help ensure that health conditions are reported accurately and managed effectively, contributing to public health efforts in Maryland.
Key takeaways
When filling out the Maryland Confidential Morbidity Report form, it’s crucial to keep a few key points in mind:
- Confidentiality is paramount. This report is designed to protect patient privacy. Ensure that all information is handled securely.
- Accurate patient information is essential. Double-check details like the patient's name, date of birth, and contact information to avoid any errors.
- Use the correct form. This form is specifically for physicians and healthcare providers. Laboratories must use different forms (DHMH 1281 & DHMH 4492).
- Report all relevant conditions. Include all diseases and conditions, as well as any laboratory results. This helps public health officials respond effectively.
- Follow up if needed. After submitting the report, be prepared for your local health department to reach out for additional information or clarification.
By keeping these points in mind, you can ensure that the reporting process is smooth and effective for both you and the public health system.
Misconceptions
Misconceptions about the Maryland Confidential Morbidity Report form can lead to confusion among healthcare providers. Here are seven common misconceptions clarified:
- Only laboratories need to report cases. This is incorrect. The form is specifically designed for use by physicians and other healthcare providers, not laboratories. Laboratories have their own designated forms.
- All patient information is public. The Maryland Confidential Morbidity Report is confidential. Patient details are protected to maintain privacy and are only shared with local health departments for public health purposes.
- Only certain diseases need to be reported. In fact, the form covers a wide range of diseases and conditions. Providers should report any condition that meets the criteria outlined in the reporting guidelines.
- Reports must be submitted immediately. While timely reporting is encouraged, there is a specific timeframe within which reports should be submitted. Providers should check local guidelines for exact deadlines.
- Patients must consent before reporting. Consent is not always required for reporting certain communicable diseases. Providers should be aware of the legal requirements regarding patient consent for reporting.
- The form is only for infectious diseases. This is a misconception. The form can also be used to report conditions related to chronic diseases and other health issues that may impact public health.
- Providers can complete the form without any training. While the form is straightforward, training is recommended. Understanding the nuances of the form can help ensure accurate and complete reporting.